CHLA pediatric neurosurgeon Peter Chiarelli, MD, DPhil, and team are using nanoparticles to deliver more precise tumor therapy.
Can a Tiny Particle Make a Big Impact on Diffuse Intrinsic Pontine Glioma?
It’s difficult to imagine just how small a nanoparticle is. A mere millionth of a millimeter in diameter, it’s roughly 20,000 times smaller than the width of a single human hair.
But tiny does not mean trivial. Nanoparticles have special properties, making them useful for everything from sunscreens to electronics to medicines. Now, a team at Children’s Hospital Los Angeles is investigating a new, potentially groundbreaking use for these minuscule molecules: enhancing radiation therapy for a devastating childhood brain tumor called diffuse intrinsic pontine glioma (DIPG).
“This would be a fundamental paradigm shift in radiation therapy,” says Peter Chiarelli, MD, DPhil, a pediatric neurosurgeon at CHLA and principal investigator on the study. “The idea is to use nanoparticles to intensify the impact of radiation against the tumor cells, while sparing the brain’s normal tissue.”
Tricking smart materials
A highly aggressive tumor deep in a child’s brainstem, DIPG grows by invasion, tightly intertwining itself with healthy brain cells. It’s impossible to surgically remove, and the survival rate is 0%.

“This cancer arises in otherwise healthy children between the ages of 5 and 9,” Dr. Chiarelli says. “Most children do not even survive a year.”
The standard of care for DIPG is radiation therapy, which can prolong survival by a few months. But while it shrinks tumors, the effect is temporary. To make this treatment more effective, Dr. Chiarelli and his research partner, Senior Scientist Meenakshi Upreti, PhD, are harnessing the power of nanoparticles.
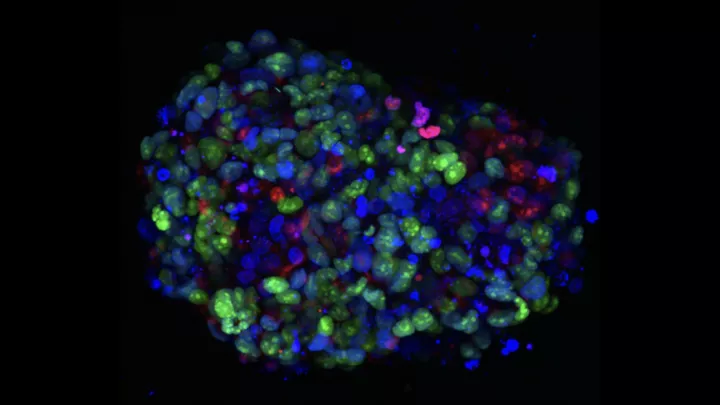
Specifically, they are developing novel nanoparticles made of tiny clusters of metals, tucked inside a biocompatible polymer shell. On the outside of that shell, they are attaching special agents that can target DIPG cells like molecular beacons. The idea is to deliver the nanoparticles only to tumor cells—and then give the radiation therapy.
“Radiation interacts with metals, and that interaction greatly intensifies its energy impact,” Dr. Chiarelli explains. “However, this greater intensity would only impact the tumor cells with the metal, not the normal, healthy tissue.
“Essentially, it’s about tricking smart materials to guide them to a specific place in the body and then deliver a much bigger punch to the cancer,” he continues. “This could allow radiation therapy to be more powerful, as well as safer.”
A novel approach
The team is currently studying different formulations of these nanoparticles and targeting agents in preclinical models. Their work is supported by a new, nearly $1 million grant from the National Institute of Neurological Disorders and Stroke, part of the National Institutes of Health (NIH).
“This is a completely new way of mobilizing nanoparticles against disease,” Dr. Chiarelli says. “Nanoparticles are already used in medicine, but mostly as a way to deliver drugs to precise targets. In this case, the nanoparticle itself—the metal—becomes the treatment when it’s combined with radiation.”
The team hopes to ultimately translate their findings into a clinical trial for patients. The lab has also benefited from a collaboration with Rex Moates, PhD, as well as philanthropic funding from private foundations and families whose children have been treated by Dr. Chiarelli.
“There’s a tremendous need for new therapies for DIPG,” Dr. Chiarelli says. “Our goal is to find those new approaches and make a difference for these children and families.”