Study Tests Therapeutic Cooling for Babies With Mild Hypoxic Ischemic Encephalopathy
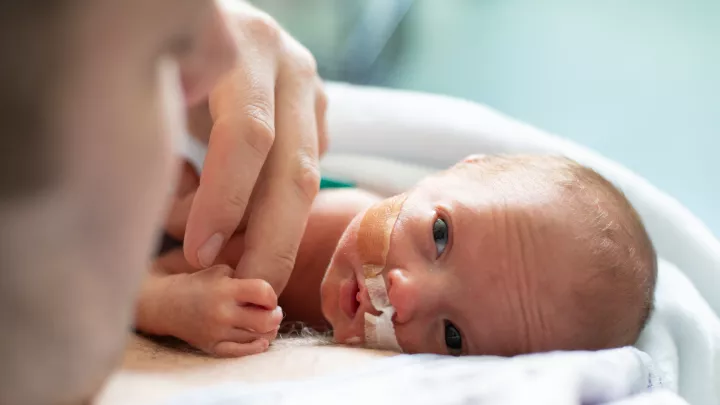
It’s well known that therapeutic hypothermia can benefit babies with moderate to severe hypoxic ischemic encephalopathy (HIE)—a brain injury caused by a lack of oxygen and blood flow that can lead to cerebral palsy and permanent brain damage. But should babies with mild HIE also receive this treatment?

That question has long been debated. Now, an observational study—open at Children’s Hospital Los Angeles—is poised to find the answer.
Called COOL Prime (Comparative Effectiveness for Cooling Prospectively Infants with Mild Encephalopathy), the prospective study is examining the effects of cooling therapy versus usual care in these newborns.
“Right now, we don’t have good evidence to support whether it’s better to cool babies with mild encephalopathy or not,” says Eni Jano, MD, a neonatologist in the Fetal and Neonatal Institute and the site principal investigator for the study at Children’s Hospital Los Angeles. “Our goal is to definitively find out if cooling should be the standard of care in these mild cases.”
How the study works

CHLA is one of 15 centers across the U.S. and Ireland that are participating in COOL Prime. Each hospital is following its standard practice of either providing cooling therapy or usual care.
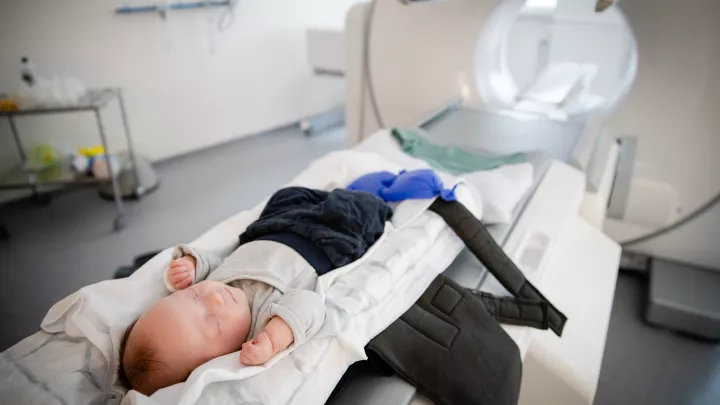
Children’s Hospital Los Angeles—long a national leader in neonatal therapeutic hypothermia—is one of seven centers in the study offering whole body cooling to newborns with mild HIE.
“We offer cooling therapy to all babies with mild encephalopathy,” notes Rachel Chapman, MD, Associate Chief of Neonatology at Children’s Hospital Los Angeles. “Families of these patients now have the option of enrolling in the study, which will help us assess the long-term impact of this treatment.”
CHLA can begin this cooling process as soon as its Emergency Transport team arrives at the hospital where the baby was born. That’s important because the therapy—which involves lowering the baby’s body temperature to 33.5 degrees Celsius (92.3 F) for 72 hours—must begin within six hours of birth.
The study is following patients for two years after treatment. “The primary goal is to see if there are differences in neurodevelopmental outcomes between babies who were cooled and those who were not,” Dr. Jano says.
Researchers will compare participants’ cognitive, language, and motor abilities using the Bayley Scales of Infant and Toddler Development, as well as parental questionnaires. The team will also assess mother-infant bonding.
A need for robust evidence
Although some earlier research has shown that babies with mild HIE who did not receive cooling therapy may later have developmental delays, those studies were too small to provide clear evidence.
The COOL Prime study aims to solve that issue by enrolling a much larger cohort—430 patients across the 15 sites.
Dr. Jano notes that whole body cooling can come with side effects—such as bradycardia (slow heart rate) and coagulopathy (trouble with blood clotting). There is also a question of whether it impacts mother-baby bonding.
While the neuroprotective benefits of cooling for moderate and severe HIE far outweigh those risks, it’s not clear if that holds true for babies with mild cases.
“We’re excited to participate in this study because it should finally give us the answer to this question,” says Dr. Jano. “Having a clear standard of care will help ensure that we are giving these babies the best chance for the most optimal outcome.”