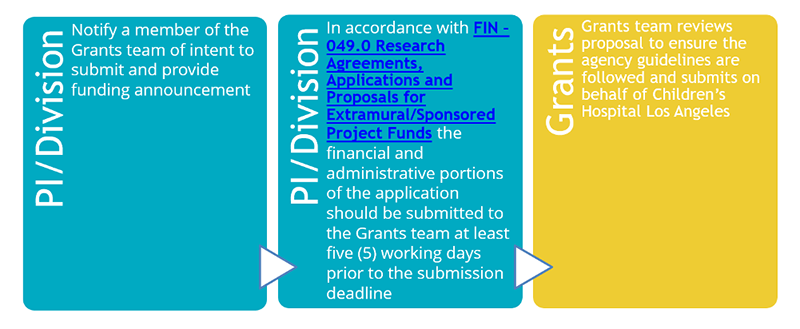
About Us
The Grants team, within the centralized department of Research Operations, is a vital resource for the research endeavors at CHLA by providing support and guidance to investigators and staff within the research community through the review, approval and submittal of all proposals as well as acceptance of awards for sponsored projects.
How We Support
While the Grants team primarily reviews, approves and submits all proposals and accepts awards on behalf of investigators at CHLA, the team also provides assistance in processing amendments and subawards, Just-In-Time (JIT) information requests, and helps with progress report reviews and submissions. Additionally, the Grants team is responsible for interpreting and advising on sponsor guidelines, applicable federal, state and local laws and regulations as well as CHLA policy and procedures.
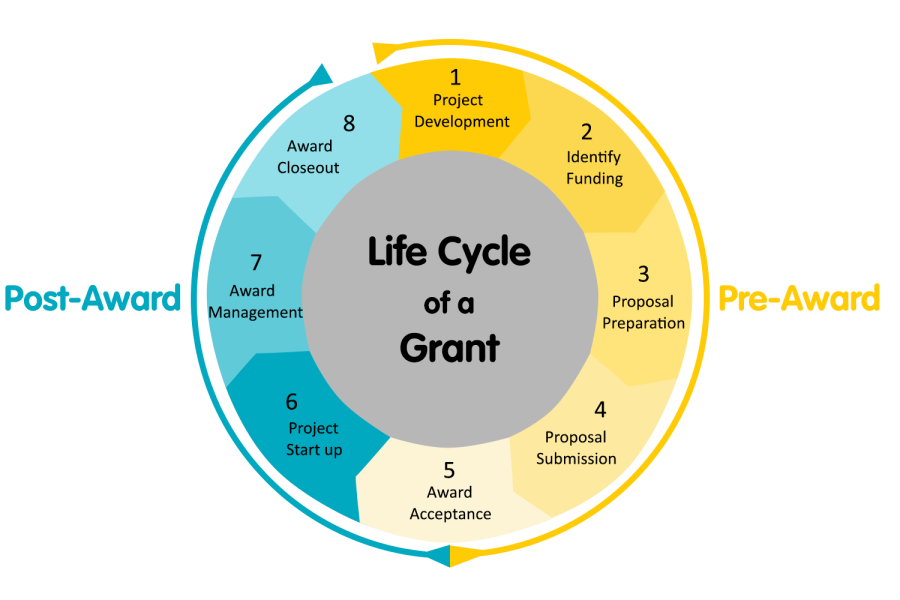
Grants Support Through the Life Cycle of a Grant
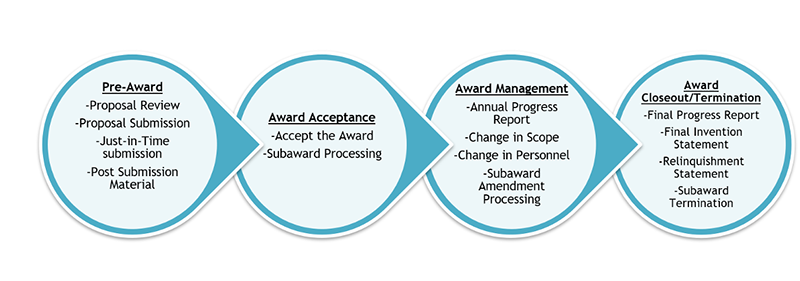
Proposal Review and Submission Life Cycle