New Approach to Modeling Ewing Sarcoma Leads to a Novel Discovery
Ewing sarcoma is an aggressive bone cancer that affects approximately 250 children and teens each year. Although many patients are cured, treatments are intense and can have long-lasting effects. In addition, about 25% of patients have tumors that spread throughout the body—resulting in poor survival.
Scientists have long sought to develop more effective and less toxic therapies for this cancer, but there has been a stumbling block: the lack of a reliable animal model system for studying the disease. Now, researchers at Children’s Hospital Los Angeles have developed such a model—in zebrafish.
“One of the big challenges in Ewing sarcoma research has been that we haven’t had a preclinical model,” explains Elena Vasileva, PhD, MSc, a postdoctoral fellow in the lab of James Amatruda, MD, PhD, at Children’s Hospital Los Angeles. “Our new zebrafish model allows us to better study the mechanisms of Ewing sarcoma development and how tumor cells are interacting with their environment.”
Those studies are already shedding new light on how Ewing sarcoma tumors grow. In a recent paper published in eLife, the researchers describe their new model—and the discovery of a pathway that could open the door to future targeted therapies.
What drives tumor growth?
Ewing sarcoma is driven by a “fusion oncogene”—basically, pieces from two different chromosomes that break off and then abnormally fuse together. The most common fusion oncogene found in Ewing sarcoma is EWSR1-FLI1. This powerful oncogene is toxic to most cell types, making it very challenging to study in other organisms.
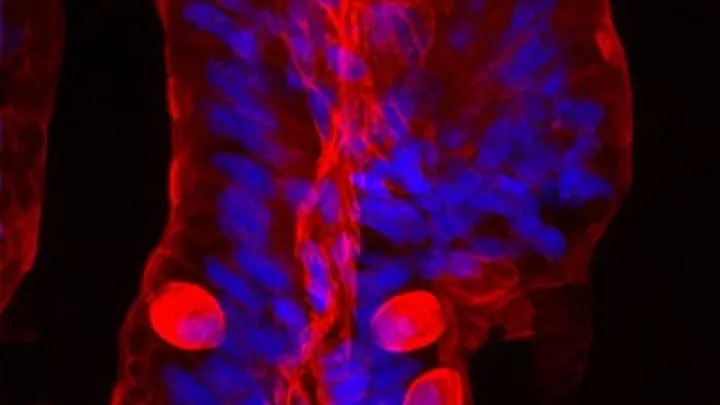
But the team overcame this challenge in zebrafish. The resulting model is very similar to human tumors and opens up a wealth of opportunities for researchers to study this cancer. Zebrafish also have the advantage of being transparent, allowing investigators to observe tumors from their earliest stages using innovative imaging techniques.
“It’s revolutionized our approach as a laboratory,” says Dr. Amatruda, senior author of the paper and Head of Basic and Translational Research for the Cancer and Blood Disease Institute. “We can now look in an unbiased way to find unexpected new insights into what drives tumor growth.”
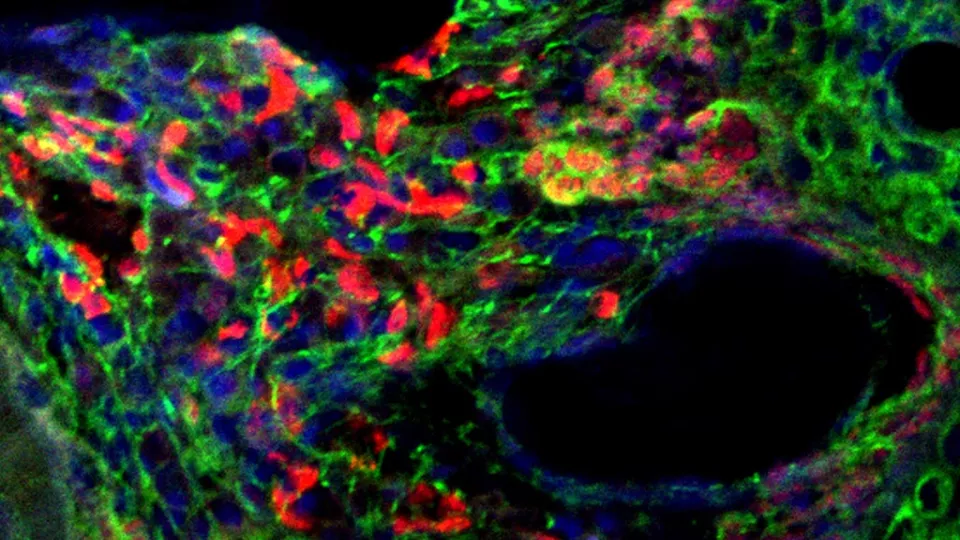
In the team’s newest study, Dr. Vasileva analyzed tissue from early-stage tumors in zebrafish and tissue from healthy fish. She then compared which proteins the cells were switching “on” in each group.
She found that the tumor cells had an abnormal extracellular matrix. This is a network of collagen, proteins and other molecules that normally surrounds a cell and acts as a support structure, helping the cell to function and stay healthy.
The Ewing sarcoma cells had exploited this matrix to create a “tumor microenvironment” that supports their survival. Specifically, Dr. Vasileva discovered that proteoglycans—molecules in the matrix that play a key role in controlling cell signaling—were switched “on” and helping to drive growth of tumor cells.
What’s more, she found that treating the cancer with a molecule called surfen, which blocks proteoglycan activity, dramatically reduced tumor cell growth.
“This is a very exciting finding because we showed that we can manipulate and inhibit cancer cell growth by blocking signaling from the cell surface,” says Dr. Vasileva, the paper’s lead author. “No one had really been studying proteoglycans in Ewing sarcoma. This has opened up many new possibilities to explore.”
Narrowing the search
The next step for the team is to probe deeper into this pathway. Because many normal tissues in the body rely on proteoglycans, it is not as simple as blocking them altogether.
Instead, the group is searching for a more specific target—an enzyme or other molecule involved in the process—that could turn off this growth signal in Ewing sarcoma, but not in normal cells.
“One of the important things about this finding is that it suggests an additional way of attacking this cancer,” says Dr. Amatruda, who holds the Dr. Kenneth O. Williams Chair in Cancer Research. “Instead of killing the tumor cells directly, we could potentially disable them from the outside—from their environment.”
The lab is using its new zebrafish model to examine other critical questions, too, including: Where do Ewing sarcoma cells come from? And how does the immune system interact with the cancer?
The research is supported by National Institutes of Health funding, but the team is also grateful for contributions from families of children with Ewing sarcoma—who provided invaluable seed funding early in this project.
In addition, the team’s ongoing studies will be made possible by the new Alfred E. Mann Family Foundation Zebrafish Laboratory at Children’s Hospital Los Angeles, which is led by Dr. Amatruda and will eventually house 120,000 zebrafish.
“Having a living model opens up so many new potential avenues for discovery,” Dr. Amatruda says. “Our goal is to continue to explore these pathways to develop more targeted and effective therapies for these young patients.”
Learn more about our Research.