Research Topics
- Metabolic disturbances associated with HIV infection and/or its treatment.
- Complications of and new treatments for congenital adrenal hyperplasia.
- The use of long-acting growth hormone to promote linear growth in short children.
Research Overview
The research of Mitchell Geffner, MD, is clinical/translational in nature and focuses on metabolic abnormalities associated with overweight and excess body fat in patients with HIV infection or with in utero maternal exposure to anti-retroviral therapy; complications of androgenic programming in patients with congenital adrenal hyperplasia; new treatments, including gene therapy, for patients with congenital adrenal hyperplasia; and use of once-weekly growth hormone to treat children with GH deficiency and other causes of short stature. Published or accepted peer-review publications from 2021 involve the following:
- Post-intervention effects of varying treatment arms on glycemic failure and beta-cell function in the TODAY study.
- Prevention of growth failure in Turner syndrome: Long-term results of early growth hormone treatment in the “Toddler Turner” cohort.
- Perinatally acquired HIV infection is associated with abnormal blood mitochondrial function during childhood/adolescence.
- Gestational diabetes in women living with HIV in Botswana: Lower rates with dolutegravir- than efavirenz-based antiretroviral therapy.
- White matter microstructural differences in youth with classical congenital adrenal hyperplasia.
- Kids N Fitness Junior: Outcomes of an evidenced-based adapted weight-management program for children ages 3-7 years.
- Metabolic syndrome and neurocognitive function in youth with perinatally-acquired HIV and youth who are HIV-exposed uninfected.
- Low adrenomedullary function predicts acute illness in infants with classical congenital adrenal hyperplasia.
- Distinct cord blood C-peptide, adipokine, and lipidomic signatures by in utero HIV exposure
Research Accomplishments
Leading-edge diagnostic testing and technology
- Use of 3T multi-shell diffusion-weighted magnetic resonance brain scans in patients with congenital adrenal hyperplasia.
- Facial structure characterization using deep learning and artificial intelligence to be able to predict who is affected with congenital adrenal hyperplasia.
Major Accomplishments
- Completion of the 15-year TODAY and TODAY 2 trials (and serving as the local PI for the entire duration) of patients with youth-onset type 2 diabetes with the recent revelation that the risk of complications, including microvascular complications, increased steadily over time and affected most participants by the time of young adulthood; complications were more common among participants of minority race and ethnic group and among those with hyperglycemia, hypertension, and dyslipidemia.
- Serving as national endocrinology consultant to the multi-center PHACs study evaluating: (1) the long-term safety of fetal and neonatal exposure to prophylactic anti-retroviral chemotherapy, as recommended by current U.S. PHS guidelines, and (2) the effects of perinatally-acquired HIV infection in adolescents.
- Discovering that facial morphologic features in patients with congenital adrenal hyperplasia are distinct and that deep learning can be used to predict CAH.
Research Goals
- To quantify the effects of intrauterine exposure to anti-retroviral therapy on growth and mitochondrial disorders in non-HIV-infected offspring and to continue longitudinal studies of endocrinological, metabolic, and mitochondrial disturbances in adolescents with perinatally acquired HIV infection.
- To further identify the long-term systemic complications of congenital adrenal hyperplasia.
- To improve treatment and outcomes of congenital adrenal hyperplasia through the use of new therapeutic medications.
- To initiate gene therapy as a potential cure for patients with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency.
- To broaden the applications of long-acting growth hormone in the treatment of pediatric growth disorders.
Key Findings
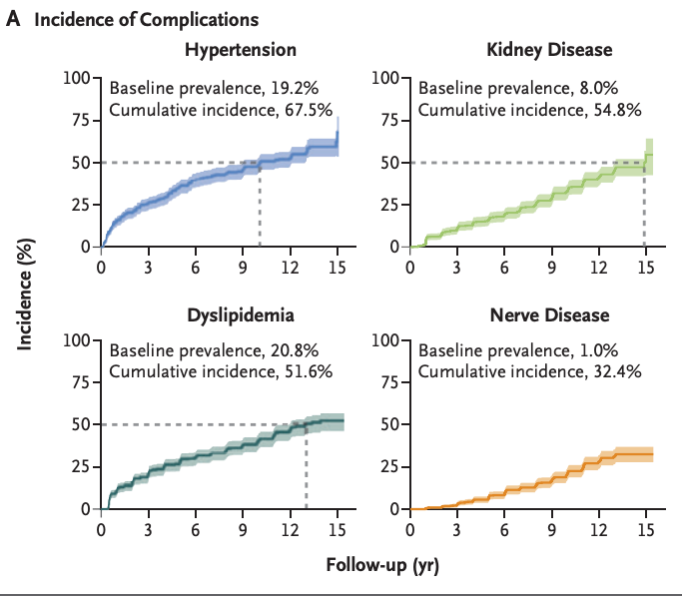
- In young adults with youth-onset type 2 diabetes, the prevalence of hypertension at baseline was 19.2%, and the cumulative incidence at 15 years was 67.5%. Dyslipidemia was present in 20.8% of the participants at baseline, and the cumulative incidence at 15 years was 51.6%. The prevalence of retinal disease, including more advanced stages, was 13.7% in the period from 2010 to 2011 and 51.0% in the period from 2017 to 2018. At least one complication occurred in 60.1% of the participants, and at least two complications occurred in 28.4%. Risk factors for the development of complications included minority race or ethnic group, hyperglycemia, hypertension, and dyslipidemia. This represents a much more virulent form of the disease compared to youth-onset Type 1 diabetes and adult-onset type 2 diabetes.
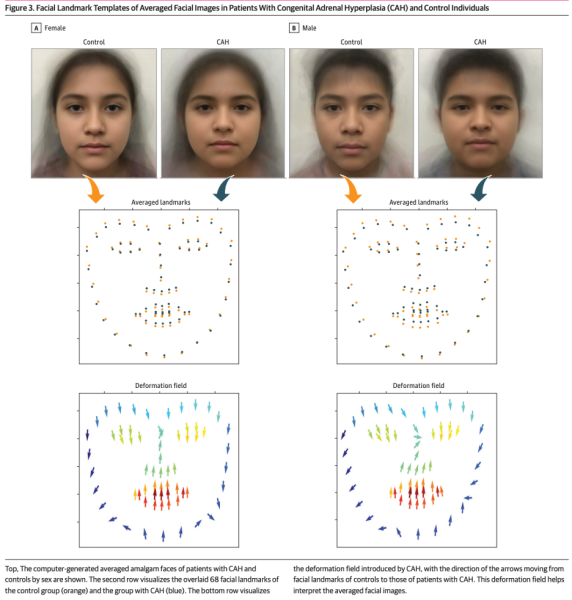
- We examined the computer-generated amalgam face image of one female and one male per group (congenital adrenal hyperplasia and control). We found on deformation analysis that there was deviation of facial landmarks in patients with CAH compared with sex-matched controls.
Current Funding
For our congenital adrenal hyperplasia studies , we rely on NIH support. Additional funding is also derived from the California Newborn Screening Program, philanthropy, and various pharmaceutical companies for various company-sponsored clinical trials,
- Effects of Androgen Excess on Lipid Distribution in Congenital Adrenal Hyperplasia
To delineate androgen effects on regional adipose depots and ectopic fat non-invasively by magnetic resonance (MR)-based imaging techniques in youth with CAH. - Abiraterone Acetate in Children with Classic 21-Hydroxylase Deficiency
To: (1) determine the minimum effective dose of abiraterone acetate that normalizes androstenedione levels in prepubertal children with CAH secondary to 21-hydroxylase deficiency (Phase 1 trial), and (2) assess the utility of abiraterone acetate in prepubertal children with CAH as adjunctive therapy to minimize excessive androgen secretion and allow more physiological glucocorticoid replacement (Phase 2 trial). - California Department of Health Services
To provide diagnosis, treatment, and outcome data for endocrine patients identified through the California Newborn Screening Program. - A Trial Comparing the Effect and Safety of Once Weekly Dosing of Somapacitan with Daily Norditropin® in Children with Growth Hormone Deficiency.
To compare the height-promoting effects of two medicines for children who do not have enough growth hormone to grow: somapacitan given once per week (a new medicine) and Norditropin® given once per day (the medicine doctors can already prescribe); the study will also test if somapacitan is safe.



