Protecting Newborn Brains
CHLA’s team of nurses and medical professionals administer hypothermia treatments to newborns to prevent brain damage caused by an inhibited oxygen supply to the brain.
Approximately 60% of prematurely born infants and 20 in every 1000 full term infants suffer from a lack of oxygen going to the brain – a condition known as hypoxic-ischemic encephalopathy or HIE – which can cause significant brain damage. In a unique study published in the Journal of Cerebral Blood Flow and Metabolism, researchers at Children’s Hospital Los Angeles have confirmed the neuroprotective effects of hypothermia as a treatment for HIE.
Therapeutic hypothermia or targeted cooling of the brain is the first therapy for neuroprotection in newborns with HIE. Without treatment, these babies often develop cerebral palsy or other severe complications. World-wide, nearly one million babies will die and another million will be left with disabilities as a result of HIE.
“There is more that we can do,” said the study’s first author, Jessica Wisnowski, PhD, of the Department of Radiology at CHLA. “Hypothermia is a first step, and with it we have been able to almost double the chance of a healthy outcome for newborns with HIE. However, about half of infants with HIE don’t adequately respond to therapy. Although we know hypothermia helps, we don’t fully understand how it helps or how best to help those babies for whom hypothermia isn’t enough to alleviate their brain injury.”
According to the researchers, better understanding of the impact of hypothermia could allow for a more tailored approach for its delivery, foster the development of early biomarkers, and direct additional neuroprotective therapies toward babies who are most likely to benefit.
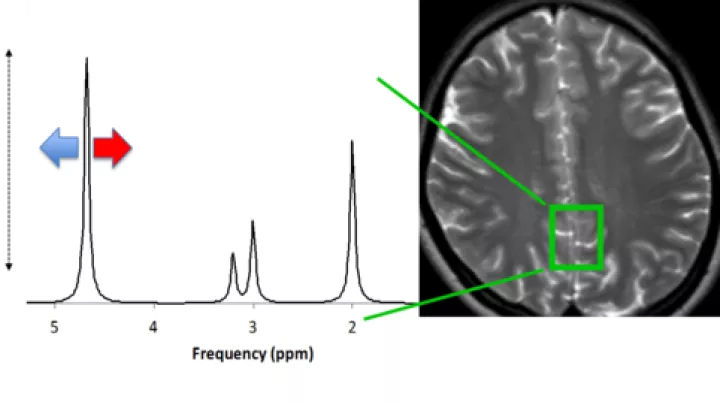
Wisnowski, principal investigator Stefan Bluml, PhD, and colleagues from both the Department of Radiology and the Newborn and Critical Care Unit (NICCU) at CHLA conducted magnetic resonance spectroscopy (MRS) studies on 31 newborn infants with HIE during hypothermia and again after the infants were re-warmed.
The team of researchers, physicians and nurses transferred the babies and all of the cooling equipment to the MRS lab – in essence “bringing the whole NICCU down to the MRI suite,” which, according to Wisnowski, enabled them to map what is happening biochemically in the brains of the infants while they were undergoing therapy.
The researchers measured the concentrations of key molecules involved in the use and storage of energy, neurotransmission and oxidative stress in newborns with HIE. Their findings demonstrated that neuroprotection is achieved by realizing a specific balance between energy metabolism and neurotransmission.
Physicians have known for a few decades that brain injury from HIE is an evolving process, disrupting metabolism of mitochondria, the “powerhouses” of the cell. It is precisely because the underlying processes that lead to cell death in the brains of newborn infants with HIE can take days, if not weeks, to develop that scientists have an opportunity to intervene with therapeutic hypothermia and save brain cells.
Wisnowski explained add that it’s paradoxical that hypothermia works because “we are slowing down brain metabolism at the same time that the brain is trying to repair itself.” In other instances in which hypothermia helps protect the brain – for instance, when a person drowns in cold water or when the body is cooled during heart surgery – brain metabolism is being slowed at the same time that the brain is vulnerable to injury, not afterwards.
The new study offers a solution to this paradox. It suggests that a key effect of hypothermia is not only a reduction in energy metabolism, but also a reduction in the synthesis of glutamate and other excitatory neurotransmitters.
“There are two functions of energy metabolism: first, metabolism generates the energy that cells need to survive; and second, energy metabolism is used to generate glutamate and other neurotransmitters – molecules that in healthy brains generate brain activity, thus contributing to the cell’s utilization of energy,” said Bluml, adding that the decrease in excitatory transmitters is expected to decrease the likelihood of seizures, but should also reduce energy utilization, since neurotransmission consumes between one-third and one-half of the brain’s energy.
Funding was provided by research grants from the Gerber Foundation and the Rudi Schulte Research Institute.